Introduction: Allogeneic stem cell transplant (allo-SCT) plays a key role in the post-remission therapy for acute myeloid leukemia (AML) patients due to its high rates of efficacy as compared to alternate therapies. For patients with relapsed/refractory AML and those with high-risk myelodysplastic syndrome (MDS), it remains the sole curative option. However, these patients continue to have significant obstacles for successful transplant including risk for relapse of underlying disease, graft versus host disease (GVHD), and infectious complications. Outcomes of allo-SCT in these patients have improved over time with the evolution of practice of allo-SCT, including modifications of transplant conditioning regimens, supportive care, and earlier recognition of transplant complications. Our study sought to assess the trends in survival in AML and MDS patients undergoing allogeneic transplant at The Ohio State University from 1984-2018.
Methods: We analyzed data from 900 consecutive patients who received an allo-SCT (705 AML and 195 MDS). The patients were stratified into 7 different groups based on year of transplant using 5 year increments; group (gp) 1 included 1984-1988, gp 2 1989-1993, gp 3 1994-1998, gp 4 1999-2003, gp 5 2004-2008, gp 6 2009-2013, and gp 7 2014-2018. Progression free survival (PFS) and overall survival (OS) were utilized as primary end points. PFS and OS were calculated using Kaplan Meier Curves. Secondary endpoints included cumulative incidences of grade II-IV and III-IV acute GVHD (aGVHD), chronic GVHD (cGVHD), and non-relapse mortality (NRM). Cumulative incidence rates were estimated and compared using Gray's test accounting for competing risks.
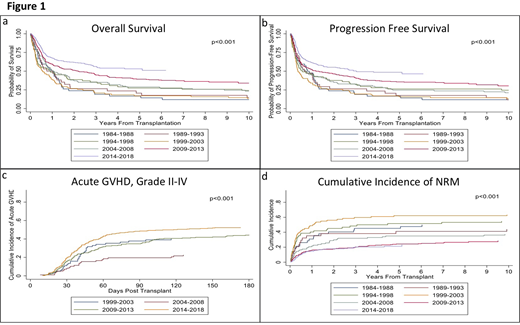
Results: Median age at transplant was 52 years (yrs) old (range 18-76) and 55.6% were male. Patients having myeloablative (MA) conditioning regimen comprised 57.6% of the cohort. From 1984 to 2018, there was a statistically significant improvement in both PFS and OS (Figure 1 a and b; p<0.001). The median PFS improved from 0.8 yrs [95% confidence interval (CI) 0.3-1.2)] in gp 1 to 3.0 yrs (95% CI: 1.7-NR) in gp 7 and the median OS from 0.9 yrs in gp 1 (95% CI:0.4-1.3) to NR (95% CI: 3.0-NR) in gp 7. The 5-yr PFS among the groups was 17, 18, 26, 16, 25, 36 and 49%, respectively, with a significant improvement seen since 2004. Similar improved trends were seen at 10 yrs. The 5-yr OS were 17, 21, 27, 17, 29, 39 and 53%, respectively, with similar significant improvement seen since 2004. Similar improved trends were also seen at 10 yrs. There was also a significant difference between rates of grade II-IV aGVHD between the groups (p<0.001), with a cumulative incidence at day +180 of 40% in gp 4, 23% in gp 5, 45% in gp 6, and 53% in gp 7 (Figure 1c). Grade III-IV aGVHD at day +180 were respectively 23, 6, 14, and 18% with the highest rate seen for gps 4 and 7. Rates of cGVHD also varied between the groups (p=0.002) with cumulative incidence at day +365 of 33% in gps 4 and 5, 38% in gp 6, and 48% in gp 7. Extensive cGVHD at day +365 increased over the years at 22, 27, 36 and 43% respectively (p<0.001). The rate of NRM significantly improved across the years, with 1-yr NRM at 31, 35, 40, 49, 22, 16 and 15% and 5-yr NRM at 45, 41, 52, 62, 35, 24 and 21% respectively, with a significant improvement seen since 2004 (p<0.001, Figure 1d).
Conclusion: Our study demonstrates significant improvement over the past several decades in survival in AML and MDS patients undergoing allo-SCT. Major factors that likely contribute to improvement in outcomes throughout the years include adjustments in conditioning regimens and GVHD prophylaxis, earlier recognition of complications as well as improved management, and improved general supportive care. Overall, while outcomes have improved significantly throughout the years, post-transplant relapses remains the leading cause of transplant failure in this group. Preventing relapse post-transplant represents a continued target for research today.
Chaudhry:Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees. Bumma:Amgen: Speakers Bureau; Sanofi: Speakers Bureau. Khan:Amgen: Consultancy; Janssen: Consultancy. Devarakonda:Janssen: Consultancy. Vasu:Omeros: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Kiadis Inc: Other: Kiadis has obtained exclusive licensing requirements from The OHio State University. Jaglowski:CRISPR: Consultancy; Novartis: Consultancy, Research Funding; Juno: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding. William:Kyowa Kirin: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Guidepoint Global: Consultancy; Incyte: Research Funding; Seattle Genetics: Research Funding; Merck: Research Funding; Dova: Research Funding. Mims:Novartis: Speakers Bureau; Agios: Consultancy; Leukemia and Lymphoma Society: Other: Senior Medical Director for Beat AML Study; Abbvie: Membership on an entity's Board of Directors or advisory committees; Kura Oncology: Membership on an entity's Board of Directors or advisory committees; Syndax Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Other: Data Safety Monitoring Board. Brammer:Seattle Genetics, Inc.: Speakers Bureau; Celgene Corporation: Research Funding. Efebera:Celgene: Research Funding; Pharmacyclics: Research Funding; Takeda: Honoraria, Speakers Bureau; Ohio State University: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.